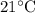 is the final river temperature after the cooling water and river have mixed.
is the final river temperature after the cooling water and river have mixed.
Step-by-step explanation:
Final river temperature after mixing using energy energy balance equation
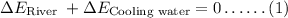
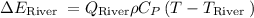
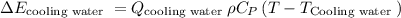
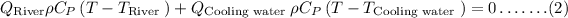
Where,
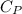 is specific heat at constant pressure,
is specific heat at constant pressure,
 change in temperature,
change in temperature,
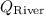 is flow in the river,
is flow in the river,
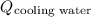 is the flow of cooling water from plant,
is the flow of cooling water from plant,
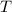 final required temperature after mixing cooling water and river water,and,
final required temperature after mixing cooling water and river water,and,
 is density of water.
is density of water.
From Diagram
While substituting,
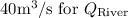
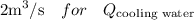
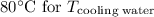 and
and
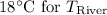
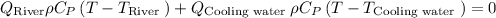
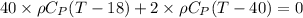
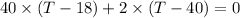
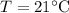
Finally, the temperature after the mixing of cool water and river is
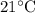 .
.