Answer:
⇛Graphite is soft in nature
⇛Diamond is hardest substance
⇛Graphite is a good conductor
⇛Diamond is not a conductor
⇛Graphite is grey in colour
⇛Diamond have transperant look
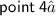
⇛Graphite have SP² hybridization
⇛Diamond have SP³ hybridization
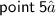
⇛Graphite have layered structure
⇛Diamond have crystalline structure