Answer:
9.24% the percent of Mg in the mixture.
Step-by-step explanation:
Mass of silver bromide = 1.1739 g
Moles of silver bromide =
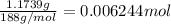
1 mole of AgBr has 1 mole of bromine atom.Then 0.006244 moles AgBr has 0.006244 mole bromine atom.
Moles of bromine atoms = 0.006244 moles
Magnesium bromide has 2 moles of bromine atom.Then 0.006244 moles of bromine atoms will be in :
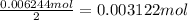
Magnesium bromide has 1 mole magnesium atom, then 0.003122 moles of magnesium bromide will have :
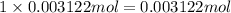 magnesium atom
magnesium atom
Mass of 0.003122 moles of magnesium :
0.003122 mol × 24 g/mol = 0.07493 g
Mass of the sample = 0.8107 g
Percentage of magnesium in the sample ;
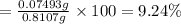
9.24% the percent of Mg in the mixture.