Answer:
maximum mass of sodium sulphate=132g
Step-by-step explanation:
firstly balance the chemical reaction,
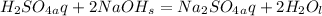
molecular weight of H2SO4=98g/mole;
Molecular weight of NaOH=40g/mole;
number of mole of H2SO4 given=91g/(98g/mole)=0.93mole;
number of mole of NaOH given=116g/(40g/mole)=2.9mole
moles are:
number of mole of H2SO4=0.93
number of mole of NaOH=2.9
From the balanced equation,
1mole of H2SO4 reacts with 2 moles of NaOH;
hence ;
0.93 mole of H2SO4 will react with 2*0.93 moles of NaOH means
moles of NaOH that reacts wih H2SO4=1.86mole but we have given 2.9 mole therefore
NaOH will be excess reagent and H2SO4 will be the limitting reagent
and mass of product depends on limitting reagent i.e mass of H2SO4.
1mole of H2SO4 produces 1 mole of Na2SO4;
0.93 mole produces 0.93 mole of Na2SO4;
maximum mass of Na2SO4 produces=mole*molecular weight;
Molecular weight of Na2SO4=142g/mole;
maximum mass of Na2SO4 produces=0.93*142=132g;
maximum mass ofNa2SO4 produces=132g;