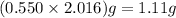Answer:
Theoretical yield of hydrogen is 1.11 g
Step-by-step explanation:
Balanced equation,
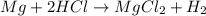
As Mg remain present in excess therefore HCl is the limiting reagent.
According to balanced equation, 2 moles of HCl produce 1 mol of
 .
.
Molar mass of HCl = 36.46 g/mol
So, 40.0 g of HCl =
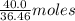 of HCl = 1.10 moles of HCl
of HCl = 1.10 moles of HCl
Hence, theoretically, number of moles of
 are produced from 1.10 moles of HCl =
are produced from 1.10 moles of HCl =
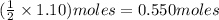
Molar mass of
 = 2.016 g/mol
= 2.016 g/mol
So, theoretical yield of
 =
=