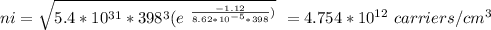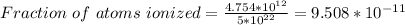Answer:
Part (i) at –70° C, intrinsic carrier concentration of silicon is 2.865 x 10⁵ carriers/cm³ and fraction of the atoms ionized is 5.37 x 10⁻¹⁸
Part (ii) at 0° C, intrinsic carrier concentration of silicon is 1.533 x 10⁹ carriers/cm³ and fraction of the atoms ionized is 3.067 x 10⁻¹⁴
Part (iii) at 20° C, intrinsic carrier concentration of silicon is 8.652 x 10⁹ carriers/cm³ and fraction of the atoms ionized is 1.731 x 10⁻¹³
Part (iv) at 100° C, intrinsic carrier concentration of silicon is 1.444 x 10¹² carriers/cm³ and fraction of the atoms ionized is 2.889 x 10⁻¹¹
Part (iv) at 125° C, intrinsic carrier concentration of silicon is 4.754 x 10¹² carriers/cm³ and fraction of the atoms ionized is 9.508 x 10⁻¹¹
Step-by-step explanation:
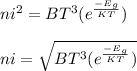
where;
B = 5.4 x 10⁻³¹
Eg = 1.12 ev
K = 8.62 x 10⁻⁵ eV/K
T = (273 + ⁰C) K
Number of atoms in silicon crystal = 5 x 10²² atoms/cm³
Part (i) For –70° C, T = (273 -70 ⁰C)K = 203 K
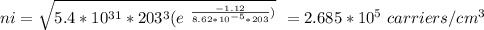
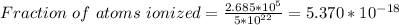
Part (ii) For 0° C, T = (273 +0 ⁰C)K = 273 K
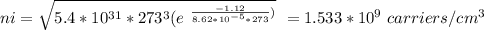
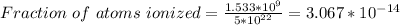
Part (iii) For 20° C, T = (273 + 20 ⁰C)K = 293 K
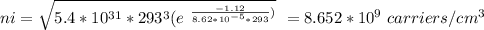
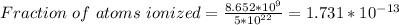
Part (iv) For 100° C, T = (273 + 100 ⁰C)K = 373 K
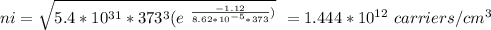
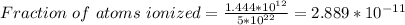
Part (v) For 125° C, T = (273 + 125 ⁰C)K = 398 K