Answer:
Therefore 373 mole of Al produce 746 mole of water.
Step-by-step explanation:
Given reaction is
3 Al+3NH₄ClO₄→Al₂O₃+AlCl₃+3NO+6H₂O
From the above reaction it is clear that 3 mole of Al produce 6 mole of water.
Therefore
3 mole of Al produce 6 mole of water.
1 mole of Al produce
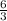 mole of water.
mole of water.
373 mole of Al produce
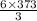 mole of water.
mole of water.
= 746 mole of water.
Therefore 373 mole of Al produce 746 mole of water.