Answer:
The rate of condensation of the steam in the condenser is 2.14 kg/s
Step-by-step explanation:
Given;
mass flow rate of water = 80 kg/s
inlet temperature of water, T₁ = 15°C
outlet temperature of water, T₂ = 30°C
temperature of the steam, ΔHvap. = 60°C
heat of vaporization of water at 60°C = 2357.7 kJ/kg
specific heat capacity of water = 4.2 kJ/kg°C
The rate of condensation can be determined by the rate of heat transfer of water as a cooling system.
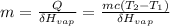
substitute the above values in this equation, we will have;
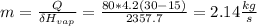
Therefore, the rate of condensation of the steam in the condenser is 2.14 kg/s