Answer:
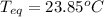
Step-by-step explanation:
Hello,
In this case, as the copper's heat loss is gained by the water, the following energetic relationship is:
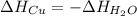
Therefore the equilibrium temperature shows up as:
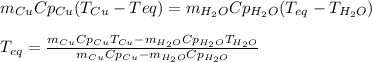
Thus, by knowing that water's heat capacity is 4.18J/g°C, one obtains:
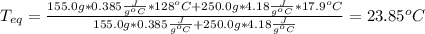
Best regards.