The given question is incomplete. The complete question is as follows.
Consider the titration of 50.0 mL of 1.00 M
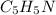 by 0.500 M HCl. For each volume of HCl added, decide which of the components is a major species after the HCl has reacted completely. Kb for
by 0.500 M HCl. For each volume of HCl added, decide which of the components is a major species after the HCl has reacted completely. Kb for
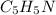 =
=
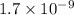 . Calculate the pH at the equivalence point for this titration.
. Calculate the pH at the equivalence point for this titration.
Step-by-step explanation:
Reaction equation of the given reaction is as follows.
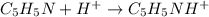
Only
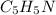 is present in the solution before the addition of HCl. Hence, the equation will be as follows.
is present in the solution before the addition of HCl. Hence, the equation will be as follows.
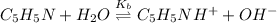
And, at the equivalence point entire
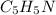 will completely react with the HCl. Hence, the solution contains
will completely react with the HCl. Hence, the solution contains
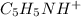 at the equivalence point. It is acidic with a pH less than 7.
at the equivalence point. It is acidic with a pH less than 7.
No. of moles of HCl = No. of moles of
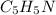
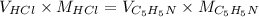
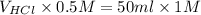
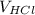 = 100 ml
= 100 ml
Hence, the volume of HCl at the equivalence point is 100 ml.
Also,
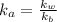
=
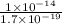
=
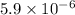
Therefore, concentration of the acid is calculated as follows.
Concentration =
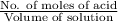
=
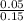
= 0.333 M
Since,
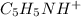 is a weak acid. So,
is a weak acid. So,
![[H^(+)] = \sqrt{k_(a) * C}](https://img.qammunity.org/2021/formulas/chemistry/college/rcbexf94g9dkv7g0x83nmsfhgu0iqdn6oa.png)
=
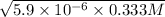
=
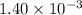
Now, we will calculate the pH of the solution at the equivalence point as follows.
pH =
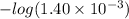
= 2.85
Thus, we can conclude that pH at the equivalence point for this titration is 2.85.