0.16 moles of Aluminum hydroxide are needed to react with 25 grams of sulfuric acid.
Step-by-step explanation:
In order to find the number of moles we first need to write down the balanced equation as,
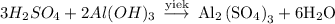
Now as mentioned above,For 3 moles of sulfuric acid , we need 2 moles of Aluminum hydroxide to balance the equation,
Thus we can balance it as,
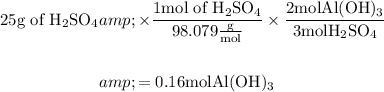
Thus it is now clearly known that 0.16 moles of Aluminum hydroxide are needed to react with 25 grams of sulfuric acid.