Answer:
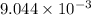 is the value of the equilibrium constant for this reaction at 756 K.
is the value of the equilibrium constant for this reaction at 756 K.
Step-by-step explanation:
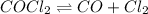
Equilibrium concentration of
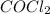
![[COCl_2]=7.40* 10^(-4) M](https://img.qammunity.org/2021/formulas/chemistry/college/vw1lkr21dx1jxtz3t6ly6hznn90ncd9i95.png)
Equilibrium concentration of
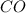
![[CO]=3.76* 10^(-2) M](https://img.qammunity.org/2021/formulas/chemistry/college/cxzq4bf86kcrqz05fx0k5crzek68mztokr.png)
Equilibrium concentration of

![[Cl_2]=1.78* 10^(-4) M](https://img.qammunity.org/2021/formulas/chemistry/college/29fkqr9t58fgwtaqy0yflt87tv780avtqr.png)
The expression of an equilibrium constant can be written as;
![K_c=([CO][Cl_2])/([COCl_2])](https://img.qammunity.org/2021/formulas/chemistry/college/hw1qnb7mczufvwk74b4d434pupyojrbqdg.png)
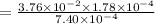
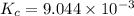
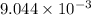 is the value of the equilibrium constant for this reaction at 756 K.
is the value of the equilibrium constant for this reaction at 756 K.