Answer:
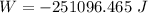 negativesign denotes thatthe work is consumed by the system.
negativesign denotes thatthe work is consumed by the system.
Step-by-step explanation:
Given:
Isothermal process.
initial temperature,
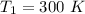
initial pressure,
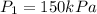
initial volume,
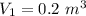
final pressure,
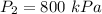
The work done during an isothermal process is given by:
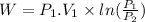
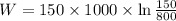
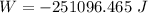 negativesign denotes thatthe work is consumed by the system.
negativesign denotes thatthe work is consumed by the system.