Answer:
composition of alpha phase is 27% B
Step-by-step explanation:
given data
mass fractions = 0.5 for both
composition = 57 wt% B-43 wt% A
composition = 87 wt% B-13 wt% A
solution
as by total composition Co = 57 and by beta phase composition Cβ = 87
we use here lever rule that is
Wα = Wβ ...............1
Wα = Wβ = 0.5
now we take here left side of equation
we will get
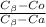 = 0.5
= 0.5
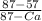 = 0.5
= 0.5
solve it we get
Ca = 27
so composition of alpha phase is 27% B