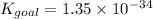Answer : The value of the equilibrium constant is,
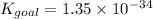
Explanation :
The given main reaction is:
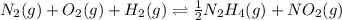
The intermediate reactions are:
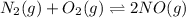 ;
;
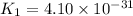
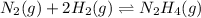 ;
;
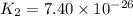
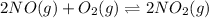 ;
;
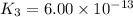
Now we are adding all the equation, we get:
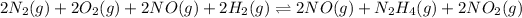
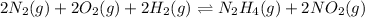 ...........(1)
...........(1)
The equilibrium constant expression will be:
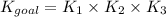
Now we are dividing equation 1 by 2, we get:
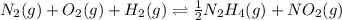 ...........(1)
...........(1)
The equilibrium constant expression will be:
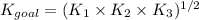
Now put all the given values in this expression, we get:
![K_(goal)=[(4.10* 10^(-31))* (7.40* 10^(-26))* (6.00* 10^(-13))]^(1/2)](https://img.qammunity.org/2021/formulas/chemistry/high-school/4fiabeb84q1wyyvracupa3medmmtdnh1vp.png)
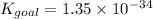
Thus, the value of the equilibrium constant is,