Answer:
The value
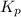 for this reaction at 1181 K is
for this reaction at 1181 K is
 .
.
Step-by-step explanation:
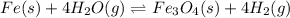
The expression of an equilibrium constant in terms of partial pressure can be given as:
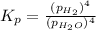
Total pressure of the gases at equilibrium = P = 69.4 Torr
Partial pressure of hydrogen gas =
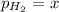
Partial pressure of water vapor =
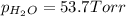
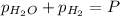 (Dalton's law of partial pressure)
(Dalton's law of partial pressure)
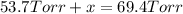
x = 69.4 Torr - 53.7 Torr = 15.7 Torr
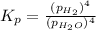
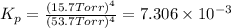
The value
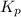 for this reaction at 1181 K is
for this reaction at 1181 K is
 .
.