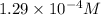Answer: The hydronium ion concentration in the solution is
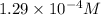
Step-by-step explanation:
To calculate the molarity, we use the equation:
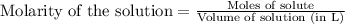
Moles hydrochloric acid solution = 0.060 mol
Volume of solution = 1 L
Putting values in above equation, we get:
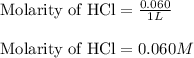
The chemical reaction for aniline and HCl follows the equation:
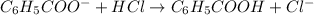
Initial: 0.24 0.060 0.31
Final: 0.18 - 0.37
To calculate the pH of acidic buffer, we use the equation given by Henderson Hasselbalch:
![pH=pK_a+\log(\frac{[\text{conjugate acid}]}{[\text{base}]})](https://img.qammunity.org/2021/formulas/chemistry/college/pl874hzfkps0hxv15h1gmkhtwnitt0bqj9.png)
![pH=pK_a+\log(([C_6H_5COO^-])/([C_6H_5COOH]))](https://img.qammunity.org/2021/formulas/chemistry/college/ijhshza4zamgl082psujl9ixdr6eufyjy9.png)
We are given:
 = negative logarithm of acid dissociation constant of benzoic acid = 4.2
= negative logarithm of acid dissociation constant of benzoic acid = 4.2
![[C_6H_5COO^-]=0.18M](https://img.qammunity.org/2021/formulas/chemistry/college/yxexl9kxzagtbcg2tc0fa9lyxqvnsy9snk.png)
![[C_6H_5COOH]=0.37M](https://img.qammunity.org/2021/formulas/chemistry/college/nhf1bbq952wp006t881wa3lk8enxgtfwau.png)
pH = ?
Putting values in equation 1, we get:
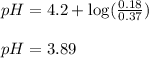
To calculate the hydronium ion concentration in the solution, we use the equation:
![pH=-\log[H_3O^+]](https://img.qammunity.org/2021/formulas/chemistry/college/svems2ww3du71jz07vrzckayblv3ponzbq.png)
pH = 3.89
Putting values in above equation, we get:
![3.89=-\log[H_3O^+]](https://img.qammunity.org/2021/formulas/chemistry/college/gokhjub7ic1di3sgf9cn4yazdpiwllsk1c.png)
![[H_3O^+]=10^(-3.89)=1.29* 10^(-4)M](https://img.qammunity.org/2021/formulas/chemistry/college/otwadgz6s4i5p0ua0wd3zrnsof80fiwvq1.png)
Hence, the hydronium ion concentration in the solution is