Answer:
Aluminium
Step-by-step explanation:
When a certain amount of heat is supplied to a substance, the temperature of the substance increases according to the equation:
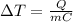
where
 is the increase in temperature
is the increase in temperature
Q is the amount of heat supplied to the substance
m is the mass of the substance
C is the specific heat capacity of the substance
Here the four substances have the same mass,
m = 50.0 g
Also, the amount of heat supplied to the 4 substances is the same. This means that their increase in temperature is inversely proportional to their specific heat capacity:
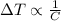
Therefore, the substance with the highest specific heat capacity will have the smallest temperature increase: by comparing the values in the table, we see that alluminium has the highest specific heat capacity (0.90 J/gC), therefore it will have the smallest temperature increase.