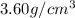Answer : The density is,
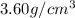
Explanation :
Formula used :
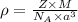 .............(1)
.............(1)
where,
 = density = ?
= density = ?
Z = number of atom in unit cell (for BCC = 2)
M = atomic mass of barium = 137.3 g/mole
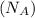 = Avogadro's number =
= Avogadro's number =
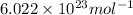
a = edge length of unit cell =
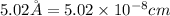
Conversion used :
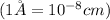
Now put all the values in above formula (1), we get
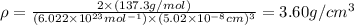
Thus, the density is,