Answer:
D) Boyle's Law
Step-by-step explanation:
Boyle's law states that:
"For a fixed mass of an ideal gas kept at constant temperature, the pressure of the gas is inversely proportional to its volume".
Mathematically, it can be written as
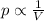
where
p is the pressure of the gas
V is its volume
This can also be written as
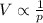
In this problem, we have a bag of potato chip which is placed on an airplane. When the plane reaches its cruising altitude, the pressure in the cabin is much lower than the pressure at sea level. As a result, also the pressure of the air inside the bag will also be lower (because the internal pressure tries to balance the external pressure).
According to Boyle's law, however, this means that the volume of the bag will increase: and at some point, the volume of the air inside the bag will be too big for the bag to "resist", and the bag will eventually explode.