Answer : The concentration of
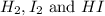 at equilibrium is, 0.244 M, 0.122 M and 1.267 M respectively.
at equilibrium is, 0.244 M, 0.122 M and 1.267 M respectively.
Explanation :
The given chemical reaction is:
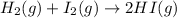
Initial conc. 0.439 0.317 0.877
At eqm. (0.439-x) (0.317-x) (0.877+2x)
As we are given:
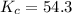
The expression for equilibrium constant is:
![K=([HI]^2)/([H_2][I_2])](https://img.qammunity.org/2021/formulas/chemistry/high-school/zigyl7hppamwc4ms5fibucq0wyyf6znfd1.png)
Now put all the given values in this expression, we get:
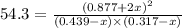
x = 0.195 and x = 0.690
We are neglecting the value of x = 0.690 because equilibrium concentration can not be more than initial concentration.
Thus, the value of x = 0.195 M
The concentration of
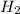 at equilibrium = (0.439-x) = (0.439-0.195) = 0.244 M
at equilibrium = (0.439-x) = (0.439-0.195) = 0.244 M
The concentration of
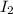 at equilibrium = (0.317-x) = (0.317-0.195) = 0.122 M
at equilibrium = (0.317-x) = (0.317-0.195) = 0.122 M
The concentration of
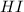 at equilibrium = (0.877+2x) = (0.877+2\times 0.195) = 1.267 M
at equilibrium = (0.877+2x) = (0.877+2\times 0.195) = 1.267 M