Answer:
1,1314.56 grams of calcium carbide can be prepared.
Step-by-step explanation:
The balanced chemical reaction ;
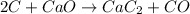
Mass of carbon present = 1.15 kg = 1150 g( 1 kg = 1000 g)
Moles of carbon =
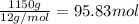
Mass of CaO present = 1.15 kg = 1150 g
Moles of CaO=
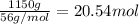
According to reaction, 2 moles of carbon reacts with 1 mole of CaO. Then 95.83 moles of carbon will react with ;
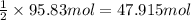 of CaO.
of CaO.
According to reaction, 1 moles of CaO reacts with 2 mole of carbon . Then 20.54 moles of carbon will react with ;
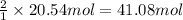 of carbon
of carbon
This means that carbon is in excess and CaO is in limiting amount ,so CaO becomes limiting reagent and amount of calcium carbide will depend upon amount of CaO.
According to reaction , 1 mole of CaO gives 1 mole of calcium carbide.Then 20.54 moles of CaO will giv e:
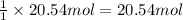 of calcium carbide
of calcium carbide
Mass of 20.54 mole of calcium carbide:
20.54 mol × 64 g/mol =1,1314.56 g
1,1314.56 grams of calcium carbide can be prepared.