Answer:
change in temperature is 142°
Step-by-step explanation:
given data
volume v = 5 m³
increase in pressure = 50 kPa
initial temperature of air = 15° C = 288 K
solution
here initial pressure P1 = Patm
initial pressure = 101325 Pa
so
final pressure will be
final pressure P2 = P1 + 50 kPa
final pressure P2 = 101.325 + 50
final pressure P2 = 151325 Pa
and
now we get final temperature so keep volume constant
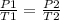 .............1
.............1
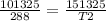
T2 = 430.11 K = 157.11°
so here change in temperature is
change in temperature = T2 - T 1
change in temperature = 157.11 - 15
change in temperature is 142°