The given question is incomplete. The complete question is as follows.
The solubility of
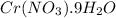 in water is 208 g per 100 g of water at
in water is 208 g per 100 g of water at
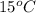 . A solution of
. A solution of
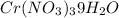 in water at
in water at
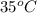 is formed by dissolving 322 g in 100 g water. When this solution is slowly cooled to
is formed by dissolving 322 g in 100 g water. When this solution is slowly cooled to
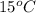 , no precipitate forms. At equilibrium, what mass of crystals do you expect to form?
, no precipitate forms. At equilibrium, what mass of crystals do you expect to form?
Step-by-step explanation:
The given data is as follows.
Solubility of
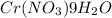 in 100 g of water = 208 g (at
in 100 g of water = 208 g (at
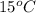 )
)
Amount of
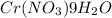 in 100 g of water = 322 g (at
in 100 g of water = 322 g (at
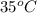 )
)
At equilibrium, the mass of crystals formed is calculated as follows.
Mass of crystal = Mass of
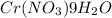 at
at
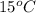 - Mass of
- Mass of
Putting the given values into the above formula, mass of the crystals will be calculated as follows.
Mass of crystal = Mass of
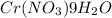 at
at
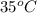 - Mass of
- Mass of
= 322 g - 208 g
= 114 g
Thus, we can conclude that crystals formed is 114 g.