Answer:
Joan should measure 0.625 mL of 2,12 M HCl solution to create her desired solution.
Step-by-step explanation:
Molarity of HCl Joan has access to =
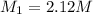
Volume of 2.12 M of HCl Joan use =
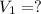
Molarity of HCl Joan desired =
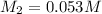
Volume of 0.053 M of HCl Joan can prepare =
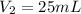

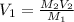
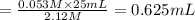
Joan should measure 0.625 mL of 2,12 M HCl solution to create her desired solution.