Complete Question
The complete question is shown on the first uploaded image
Answer:
The force on the lid when the air inside the cooker had been heated to 120°C is =
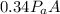
The force on the lid when the air inside the cooker had been heated to 20°C is =
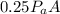
The
Step-by-step explanation:
The main concept used to solve this problem is Pressure-Temperature Law.
Initially, find the pressure inside the pressure cooker by using the Pressure-Temperature Law. Then, find the force exerted by the air inside cooker on the lid of the cooker.
Fundamentals :
The Pressure-Temperature Law states that the pressure of the of a given gas held at constant volume is directly proportional to the Kelvin temperature. The increase in pressure results in an increase in the temperature and vice-versa.
The expression of the Pressure-Temperature Law is as follows:
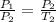
Here,
 and
and

are the initial and final pressures and
 and
and

are the initial and final temperatures respectively.
The force in terms of pressure can be given by the following expression.
F=(ΔP) A
Here, A is the area on which the pressure is applied and ΔP is the change in pressure
The step- step calculation is shown on the second,third,fourth, fifth image
Note:
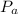 is the pressure of atmosphere
is the pressure of atmosphere