Answer:
The work done per unit kg of mass is -76.08 kJ/kg while the heat transferred per unit kg of mass is -42.01 kJ/kg.
Step-by-step explanation:
For Argon the molar mass is given as
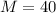
Now the gas constant for Argon is given as
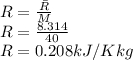
Initial Temperature is T_1=10 C=10+273=283 K
Final Temperature is T_2=120 C=120+273=393 K
Initial Pressure is P_1=120 kPa
The initial volume is given as
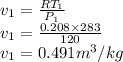
For the polytropic process with γ=1.3 is given as
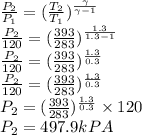
Now the volume at the second stage is given as
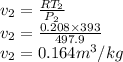
Now the work done per kg mass is given as
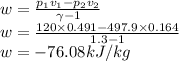
So the work done per unit kg of mass is -76.08 kJ/kg.
The heat per unit mass is given as
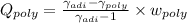
As the Argon gas is monotonic which gives γ_adiabatic=1.67
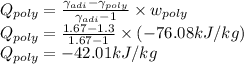
The heat transferred per unit kg of mass is -42.01 kJ/kg