Answer:
The volume will be 2600 L.
Step-by-step explanation:
Initial temperature (
 ) = 25°C = (25 + 273) K = 298K
) = 25°C = (25 + 273) K = 298K
Final temperature (
 ) = 54°C = (54 + 273) K = 327 K
) = 54°C = (54 + 273) K = 327 K
By using the combined gas law equation,
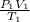 =
=
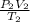
or,
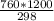 =
=
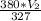
or, 3060 × 327 = 380 ×

or,
 ≈ 2600 L
≈ 2600 L
Hence the volume of the balloon will be about 2600 L.