Answer:
The answers to the question are as follows
(a) W = -175.6 MJ
(b) W = -329.256 MJ
The peak temperature of the isentropic compression process is 886.974 K
Step-by-step explanation:
(a) We are given the initial conditions as
v₂ = 10 m³
T₂ = 15 °C
p₂ (gauge) = 4.5 MPa gauge → 4.5 MPa + 1 atm = 4.5 MPa + 101325 Pa = 4.601 MPa
p₁ = 1 atm
Therefore isothermal compression we have the work done given by
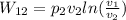 per unit mass of the given gas, hence
per unit mass of the given gas, hence
From the relation
p₁·v₁ =p₂·v₂ therefore v₁ = p₂·v₂/p₁ = 4.6 MPa× 10 m³/(1 atm) = 4.6 MPa× 10 m³/(101325 Pa) = 454.115 m³
Therefore W₁₂ = 101325 Pa × 454.11 m³× ㏑((10 m³)/(454.115 m³)) = 46013250×(-3.82) = -175575813.855 J = -175.6 MJ
W = -175.6 MJ
(b) For isentropic compression we have
W = m×cv×(T₂ -T₁)
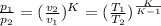
for air we put K = 1.4
therefore we have
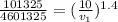 from which
from which
v₁ = 152.65 m³
We also have
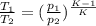 or
or
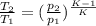 from which we find the value of T₂ as
from which we find the value of T₂ as
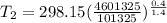 = 886.974 K (peak temperature)
= 886.974 K (peak temperature)
Therefore from pv = RT and R =cp -cv = 1.005 -0.718 = 0.287 kJ/kg·K
Therefore number of moles = pv/(RT) = (4601325×10)(287×288.15) = 556.394 kg
m = 556.394 kg
Therefore work done at constant pressure = m·cp·(T₂-T₁) gives
556.394 kg × 1.005 kJ/kg⋅K×(298.15 K-886.974 K ) = -329256.19 kJ or -329.256 MJ
The peak temperature of the isentropic process = 886.974 K