Answer:
d. Precipitation and gas evolution
Step-by-step explanation:
The reaction of aqueous solutions of barium sulfide and sulfuric acid is as follows -
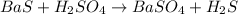
The above reaction is a type of precipitation reaction , as on reacting the two or more reactants , a precipitate is generated as one of the product , is referred to as a precipitation reaction .
The state of H₂S is gaseous in nature , and hence , a gaseous products is generated along with a precipitate ,
Hence , from the above reaction ,
The correct option is d. Precipitation and gas evolution .