Answer:
The answer to the question is
The heat transferred in the process is -274.645 kJ
Step-by-step explanation:
To solve the question, we list out the variables thus
R-134a = Tetrafluoroethane
Intitial Temperaturte t₁ = 100 °C
Initial pressure = 3.5 bar = 350 kPa
For closed system we have m₁ = m₂ = m
ΔU = m×(u₂ - u₁) = ₁Q₂ -₁W₂
For constant pressure process we have
Work done = W =
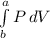 = P×ΔV = P × (V₂ - V₁) = P×m×(v₂ - v₁)
= P×ΔV = P × (V₂ - V₁) = P×m×(v₂ - v₁)
From the tables we have
State 1 we have h₁ = (490.48 +489.52)/2 = 490 kJ/kg
State 2 gives h₂ = 206.75 + 0.75 × 194.57= 352.6775 kJ/kg
Therefore Q₁₂ = m×(u₂ - u₁) + W₁₂ = m × (u₂ - u₁) + P×m×(v₂ - v₁)
= m×(h₂ - h₁) = 2.0 kg × (352.6775 kJ/kg - 490 kJ/kg) =-274.645 kJ