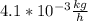Answer:
The number of kilo- grams of hydrogen that pass per hour through this sheet of palladium is
Step-by-step explanation:
Given
x1 = 0 mm
x2 = 6 mm = 6 *
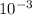 m
m
c1 = 2 kg/

c2 = 0.4 kg/

T = 600 °C
Area = 0.25
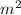
D = 1.7 *
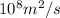
First equation
J = - D
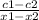
Second equation
J =
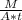
To find the J (flux) use the First equation
J = - 1.7 *
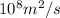 *
*
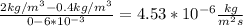
To find M use the Second equation
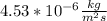 =
=
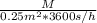
M =