Answer : The mass of
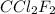 evaporated must be, 1.217 kg
evaporated must be, 1.217 kg
Explanation :
First we have to calculate the moles of water.
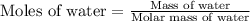
Molar mass of water = 18 g/mol
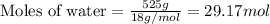
Now we have to calculate the heat released.
Heat released = Moles of water × Molar heat of fusion of ice
Heat released = 29.17 mol × 6.01 kJ/mol
Heat released = 175.3 kJ
Now we have to calculate the moles of
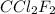
Heat = Moles of
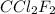 × Molar heat of vaporization of
× Molar heat of vaporization of
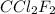
175.3 kJ = Moles of
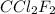 × 17.4 kJ/mol
× 17.4 kJ/mol
Moles of
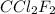 = 10.07 mol
= 10.07 mol
Now we have to calculate the mass of
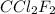
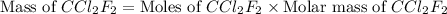
Molar mass of
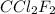 = 120.9 g/mol
= 120.9 g/mol
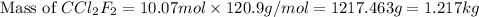
Thus, the mass of
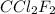 evaporated must be, 1.217 kg
evaporated must be, 1.217 kg