Answer : The volume of chloramphenicol stock solution needed is, 0.4 mL
Explanation :
First we have to calculate the mass of chloramphenicol.
As, the mass of chloramphenicol in 1 mL of solution =
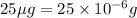
So, the mass of chloramphenicol in 400 mL of solution =
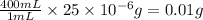
Thus, the mass of chloramphenicol is, 0.01 g
Now we have to calculate the volume of chloramphenicol stock solution needed.
Concentration of chloramphenicol =
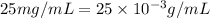
As,
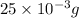 of chloramphenicol needed 1 mL volume of chloramphenicol stock solution
of chloramphenicol needed 1 mL volume of chloramphenicol stock solution
So,
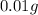 of chloramphenicol needed
of chloramphenicol needed
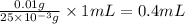 volume of chloramphenicol stock solution
volume of chloramphenicol stock solution
Thus, the volume of chloramphenicol stock solution needed is, 0.4 mL