The maximum mass of NaCl is 0.54 g.
Step-by-step explanation:
The reaction can be represented with the following equation:
HCl + NaOH ----> NaCl + H2O
According to the equation, equal amounts of chemical agents and resultant products (in moles) participate in this reaction.
The amount of hydrochloric acid is as follows:
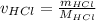
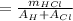
= 1.1 / (1 + 35.46)
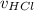 = 0.030 moles.
= 0.030 moles.
where m is the mass,
A is the atomic mass of an element.
The amount of sodium hydroxide is as follows:
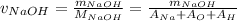
= 0.420 / (23 + 16 + 1)
= 0.0105 moles.
Since the amount of sodium hydroxide exceeds the amount of hydrochloric acid, the maximum amount of each substance participating in the reaction is v =
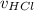 = 0.030 moles.
= 0.030 moles.
Therefore, the maximum amount of water that can be produced is as follows:
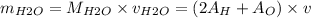
= (2
 1 + 16)
1 + 16)
 0.030 = 0.54 g.
0.030 = 0.54 g.
The maximum mass of NaCl is 0.54 g.