Answer:
-1 J/K
Step-by-step explanation:
The entropy (ΔS) of a system is the ratio of the quantity of heat (Q) exchanged (removed or added) in the system to the temperature (T) involved. i.e
ΔS =
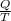 ---------------------------(i)
---------------------------(i)
Since, heat was removed from the system the quantity of heat is negative. i.e
Q = -ve
From the question;
Q = -470J
T = 470K
Substitute these values into equation (i) as follows;
ΔS =
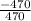 = - 1J/K
= - 1J/K
Therefore, the change in entropy of the system (reservoir in this case) is - 1 J/K