Answer:
Adduct, donate, accept
Step-by-step explanation:
Lewis acids are those having suitable vacant orbital for electron acceptance and forming a covalent bond with an electron rich species. Example:
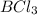 (B has vacant 2p orbital for electron acceptance)
(B has vacant 2p orbital for electron acceptance)
Lewis bases are those having energetically available unshared electrons that can be donated to an electron deficient species and form a covalent bond. Example:
 (N has energetically available lone pair)
(N has energetically available lone pair)
So, product of Lewis acid-base reaction is an adduct (
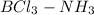 ) where a new covalent bond is formed between B and N.
) where a new covalent bond is formed between B and N.