Answer:
![([F^(-)])/([HF])](https://img.qammunity.org/2021/formulas/chemistry/college/skus0miq0jg6gx9xei9oabsdqnob3nvndl.png) is larger
is larger
Step-by-step explanation:
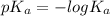 , where
, where
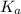 is the acid dissociation constant.
is the acid dissociation constant.
For a monoprotic acid e.g. HA,
![K_(a)=([H^(+)][A^(-)])/([HA])](https://img.qammunity.org/2021/formulas/chemistry/college/xnvrckfplsbmfv4pvkqk2ozsb3uta1q2ap.png) and
and
![([A^(-)])/([HA])=(K_(a))/([H^(+)])](https://img.qammunity.org/2021/formulas/chemistry/college/sbyr7gyk8lwbyxytkmkd82vfr1zridmi5r.png)
So, clearly, higher the
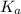 value , lower will the the
value , lower will the the
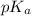
In this mixture, at equilibrium,
![[H^(+)]](https://img.qammunity.org/2021/formulas/chemistry/high-school/6hb4zpwkhtbq7b9xvc210bz7tsiz8l7l6h.png) will be constant.
will be constant.
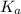 of HF is grater than
of HF is grater than
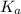 of HCN
of HCN
Hence,
![((F^(-))/([HF])=(K_(a)(HF))/([H^(+)]))>((CN^(-))/([HCN])=(K_(a)(HCN))/([H^(+)]))](https://img.qammunity.org/2021/formulas/chemistry/college/8sfq7saka98p7b2fwayc5z4127xqnu0rac.png)
So,
![([F^(-)])/([HF])](https://img.qammunity.org/2021/formulas/chemistry/college/skus0miq0jg6gx9xei9oabsdqnob3nvndl.png) is larger
is larger