Step-by-step explanation:
For AX type ceramic material, the number of formula per unit cells is as follows.
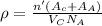
or,
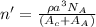
where, n' = no. of formula units per cell
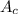 = molecular weight of cation = 90.5 g/mol
= molecular weight of cation = 90.5 g/mol
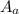 = molecular weight of anion = 37.3 g/mol
= molecular weight of anion = 37.3 g/mol
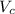 = volume of cubic cell = 3.55
= volume of cubic cell = 3.55
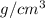
a = edge length of unit cell =
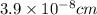
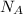 = Avogadro's number =
= Avogadro's number =
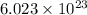
 = density = 3.55
= density = 3.55
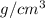
Now, putting the given values into the above formula as follows.
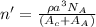
=
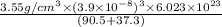
= 0.9
= 1 (approx)
Therefore, we can conclude that out of the given options crystal structure of cesium chloride is possible for this material.