Answer:
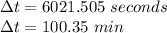
This Time is optimistic as calculated are based on ideal case. Considering the real case, refrigerated space will gain heat from surrounding and increase work load and hence requires more time to cool watermelons to required temperature.
Step-by-step explanation:
Given:
Power input=
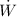 =450 W
=450 W
COP=1.55
N=5 watermelons
Mass of each watermelon=10 Kg
Initial Temperature=
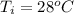
Final Temperature=
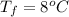
Specific heat=
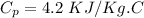
Required:
How long it will take for the refrigerator to cool watermelons?
Solution:
Heat Removed from watermelons is:
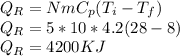
Rate of heat removed:

Time is Calculated as:

This Time is optimistic as calculated are based on ideal case. Considering the real case, refrigerated space will gain heat from surrounding and increase work load and hence requires more time to cool watermelons to required temperature.