Answer:
The answer to the question is
It will be ≅ 28.8 years until only 35 % of the substance remains
Step-by-step explanation:
To solve this we note the required relations and the given variables thus
The half life of the substance is
N(t) = N(0) ×
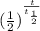
Thus when only 35% is remaining we have
35% of N(0) = N(0)×
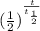
or 0.35 =
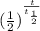 , to solve the previous equation, we take the log of both sides thus
, to solve the previous equation, we take the log of both sides thus
Log (0.35) = Log (
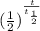 ) or
) or
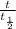 × log (1/2) =log(0.35)
× log (1/2) =log(0.35)
⇒
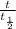 = 1.515 and since
= 1.515 and since
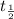 = 19 years we have
= 19 years we have
t = 19 × 1.515 = 28.776 Years
Therefore it will be 28.776 years before 35 % of the substance will be remaining