Answer:
η = 48.1 %
Step-by-step explanation:
Given that
The maximum temperature ,T(max) = 350 C
T(max) = 350+ 273 = 623 K
The minimum temperature ,T(min) = 50 C
T(min) = 50 + 273 = 323 K
We know that efficiency of Carnot cycle is given as
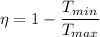
Now by putting the values in the above equation we get
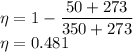
The efficiency of Carnot cycle will be 48.1 %.
Therefore the answer will be 48.1 %.
η = 48.1 %