Answer: The mass percent of water in the original air sample is 4.16 %
Step-by-step explanation:
We are given:
Volume of moist air = 12.0 L
Volume of dry air = 11.50 L
Volume of water lost = (12.0 - 11.50) L = 0.50 L
Here, the percent by mass ratio will be equal to the percent by volume ratio as the number of moles is directly related to the volume.
To calculate the volume percentage of water in the air, we use the equation:
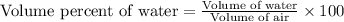
Putting values in above equation, we get:
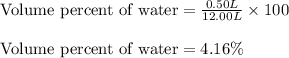
Hence, the mass percent of water in the original air sample is 4.16 %