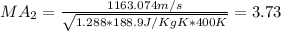Answer:
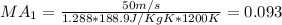
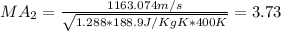
Step-by-step explanation:
Assuming this problem: "Carbon dioxide enters an adiabatic nozzle at 1200 K with a velocity of 50 m/s and leaves at 400 K. Assuming constant specific heats at room temperature, determine the Mach number (a) at the inlet and (b) at the exit of the nozzle. Assess the accuracy of the constant specific heat assumption."
Part a
For this case we can assume at the inlet we have the following properties:
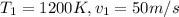
We can calculate the Mach number with the following formula:
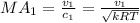
Where k represent the specific ratio given k =1.288 and R would be the universal gas constant for the carbon diaxide given by:
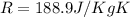
And if we replace we got:
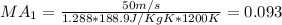
Part b
For this case we can use the same formula:
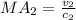
And we can obtain the value of v2 from the total energy of adiabatic flow process, given by this equation:
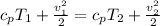
The value of
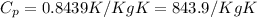 and the value fo T2 = 400 K so we can solve for
and the value fo T2 = 400 K so we can solve for
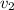 and we got:
and we got:
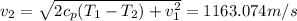
And now we can replace on this equation:
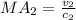
And we got: