Answer: The annual emission rate of SO2 is 1.08 ×
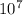 kg/yr
kg/yr
Step-by-step explanation:
- The rate r at which the coal is been burnt is 8.02 kg/s.
- Amount of sulphur in the burning coal is given as 4.40 %
i.e., 4.4/100 × 8.02 = 0.353 kg/s. Which is equivalent to the rate at which the sulphur is been burnt.
- Since the burning of sulphur oxidizes it to produce SO2, it follows that the non-oxidized portion of the sulphur will go with the bottom ash.
- The bottom ash is said to contain 2.80 % of the input sulphur.
- Hence the portion of the SO2 produced is 100 — 2.80 = 97.20 %.
- The rate of the SO2 produced is percentage of SO2 × rate at high sulphur is been burnt.
= 97.20/100 × 0.353 kg/s.
= 0.343 kg/s.
- To get the annual emission rate of SO2, we convert the kg/s into kg/yr.
1 kg/s = 1 kg/s × (60 × 60 × 24 × 365) s/yr
1 kg/s = 31536000 kg/yr
- Therefore, 0.343 kg/s = 0.343 × 31536000 kg/yr
= 10816848 kg/yr
= 1.08 × 10^7 kg/yryr.