Answer: b. there is no net change in the amount of substrates or products.
Step-by-step explanation:
The reactions which do not go on completion and in which the reactant forms product and the products goes back to the reactants simultaneously are known as equilibrium reactions.
Equilibrium state is the state when reactants and products are present but the concentrations does not change with time.
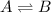
For a chemical equilibrium reaction, equilibrium state is achieved when the rate of forward reaction becomes equals to rate of the backward reaction.