Answer:
Acetic acid (
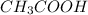 ) can release only one single H+ ion .
) can release only one single H+ ion .
Therefore we can say that acetic acid has only one pKa value.
Amino acids have one -COOH( carboxylic group) group and one -NH2 (amino group) group which can both release one single H+ ion.
Step-by-step explanation:
Acetic acid (
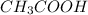 ) can release only one single H+ ion .
) can release only one single H+ ion .
Therefore we can say that acetic acid has only one pKa value.
Amino acids have one -COOH( carboxylic group) group and one -NH2 (amino group) group which can both release one single H+ ion.
In amino acids -
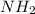 group becomes
group becomes
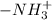 in the presence of acids.
in the presence of acids.
in the presence of bases
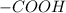 becomes
becomes
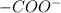
Therefore two pKa values can be observed amino acids.