Step-by-step explanation:
It is known that charge on xenon nucleus is
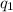 equal to +54e. And, charge on the proton is
equal to +54e. And, charge on the proton is
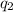 equal to +e. So, radius of the nucleus is as follows.
equal to +e. So, radius of the nucleus is as follows.
r =
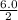
= 3.0 fm
Let us assume that nucleus is a point charge. Hence, the distance between proton and nucleus will be as follows.
d = r + 2.5
= (3.0 + 2.5) fm
= 5.5 fm
=
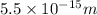 (as 1 fm =
(as 1 fm =
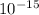 )
)
Therefore, electrostatic repulsive force on proton is calculated as follows.
F =
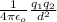
Putting the given values into the above formula as follows.
F =
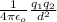
=
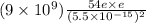
=
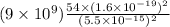
= 411.2 N
or, =
 N
N
Thus, we ca conclude that
 N is the electric force on a proton 2.5 fm from the surface of the nucleus.
N is the electric force on a proton 2.5 fm from the surface of the nucleus.