Answer:
The relation between the shielding and effective nuclear charge is given as
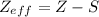
where s denote shielding
z_{eff} denote effective nuclear charge
Z - atomic number
Step-by-step explanation:
shielding is referred to as the repulsion of an outermost electron to the pull of electron from valence shell. Higher the electron in valence shell higher will be the shielding effects.
Effective nuclear charge is the amount of net positive charge that valence electron has.
The relation between the shielding and the effective nuclear charge is given as
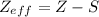
wheres denote shielding
z_{eff} denote effective nuclear charge
Z - atomic number