Answer:
0.327 M is the concentration of hydrochloric acid in the final solution.
Step-by-step explanation:
Case 1:
716.7 ml of 3.86 M HCl. Using a volumetric pipet, you take 119.56 ml of that solution and dilute it to 969.88 ml in a volumetric flask.
Before dilution :
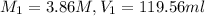
After dilution :
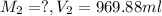
 ( dilution )
( dilution )
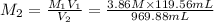
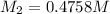
Case 2:
Now you take 100.00 ml of case 1 solution and dilute it to 145.62 ml in a volumetric flask
Before diluting it further :
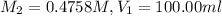
After dilution :
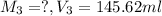
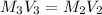 ( dilution )
( dilution )
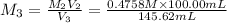
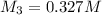
0.327 M is the concentration of hydrochloric acid in the final solution.